Hong Kong Electronics Fair (Autumn Edition)
Oct.16.24
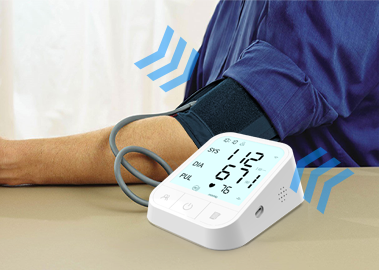
Our booth was like a treasure trove of health. There are brand new blood pressure monitors here, accurately controlling the dynamics of blood pressure and protecting your cardiovascular health.
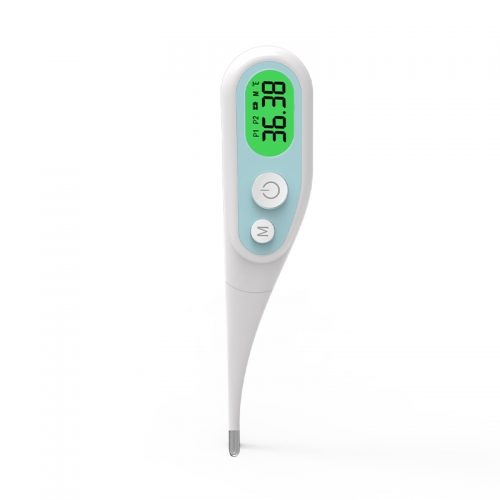
Thermometer, small but powerful, quickly and accurately meas
2024 CMEF Shenzhen
Oct.16.24
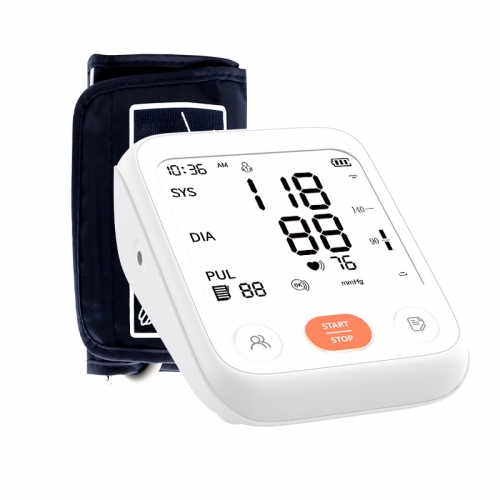
On October 12, our booth made a wonderful debut, with an accurate and reliable sphygmomanometer to protect your health at any time, a sensitive and convenient thermometer to keep an eye on your body temperature changes, and an oximeter that can quickly de
Kind + Jugend 2024
Sep.06.24
The theme of our booth is rich and varied products, including the intimate breast pump, which provides mothers with convenience and comfort; the accurate thermometer, which always guards the healthy temperature of the whole family; the practical nasal asp
AOJ Medical Unveils Cutting-Edge Mother and Baby Care Solutions at 2024 Hong Kong Baby Products Fair
Jan.12.24
At the 2024 Hong Kong Baby Products Fair, AOJ Medical unveiled a range of cutting-edge mother and baby care solutions, including advanced breast pumps, nasal aspirators, and itch relievers.
MEDICA 2023 Grand Opening, AOJ Medical at the show.
Nov.20.23
At MEDICA 2023 in Germany, AOJ MEDICAL presents our latest breast pumps and nasal aspirators. These innovative tools are the result of our deep understanding of infant and family health needs. We also offer a variety of blood pressure monitors, thermomete