|
Product name |
FDA approved 510k non-contact infrared thermometer with local medical device license |
|
|
Applicable regulations and laws
|
ASTM E 1965-98 (Reapproved 2009) Standard Specification for Infrared Thermometers for Intermittent Determination of Patient Temperature |
|
|
ISO80601-2-56 First Edition 2009-10-01 Medical Electrical Equipment - Part 2-56: Particular Requirements For Basic Safety And Essential Performance Of Clinical Thermometers For Body Temperature Measurement. (General Plastic Surgery/General Hospital) |
||
|
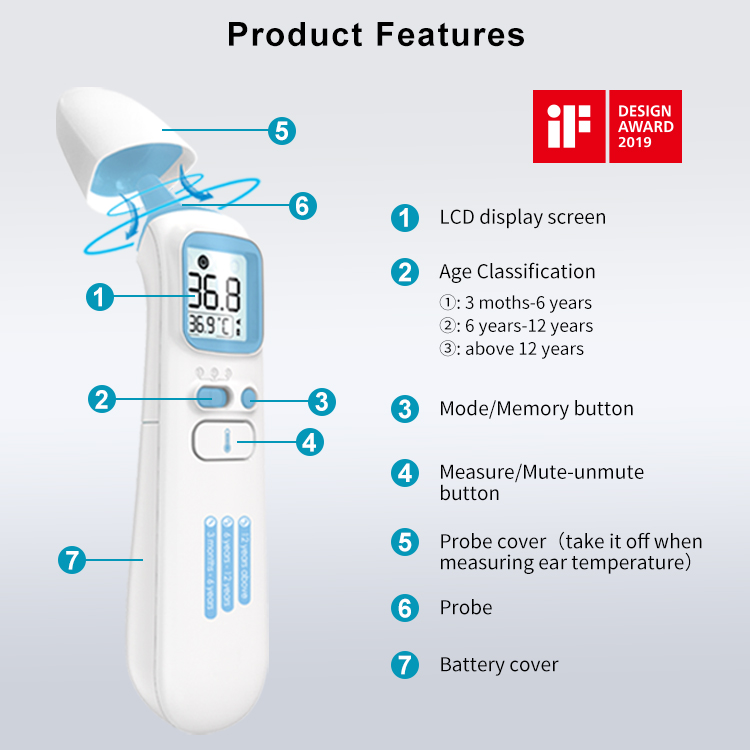
Display |
Segment LCD, green and red LCD backlight |
|
|
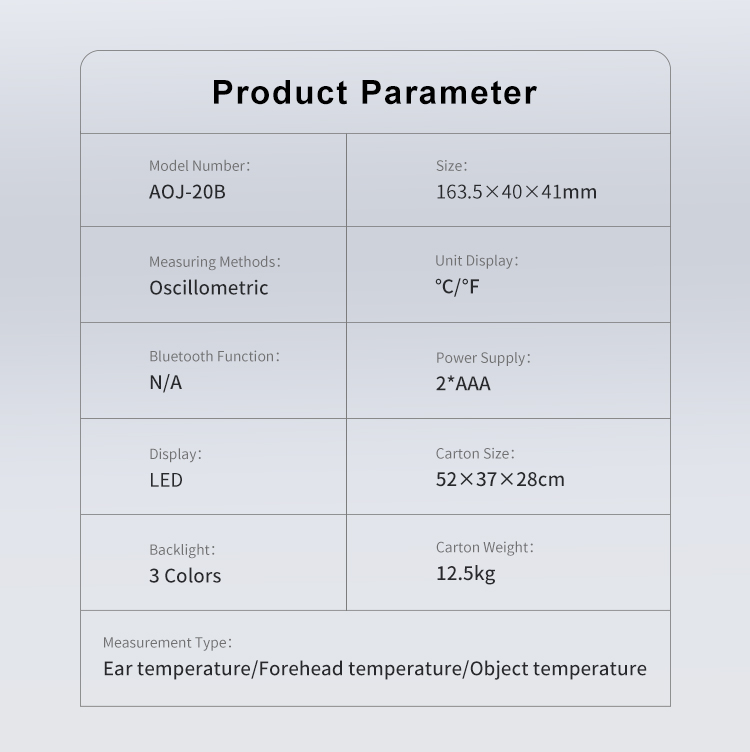
Temperature units |
℃ /℉, switchable |
|
|
Power supply |
DC 3V, AAAX2 |
|
|
Measurement range |
Forehead: 32.0°C-42.9°C (98.6°F-109.2°F) |
|
|
Ear: 32.0°C-42.9°C (98.6°F-109.2°F) |
||
|
Object: 0°C-100°C (32°F-212°F) |
||
|
Accuracy (Laboratory) |
Ear & Forehead mode |
±0.2℃ /±0.4℉ |
|
Object mode |
±1.0°C/2.0°F |
|
|
Display resolution |
0.1℃/℉ |
|
|
Automatic shutdown |
10s±1s |
|
|
Memory |
40 groups of measured temperature. |
|
|
Operational conditions |
Temperature: 10℃-40℃ (50℉-104℉) / Humidity: 15-95%RH, non-condensing Atmospheric pressure: 70-106 kPa |
|
|
Storage condition |
Ambient Temperature: -20°C to 55°C (-4°F-131°F) Relative Humidity: 0-95% RH, non-condensing Atmospheric pressure: 50kPa to 106kPa |
|
|
Battery |
2*AAA, can be used for more than 3000 times |
|
|
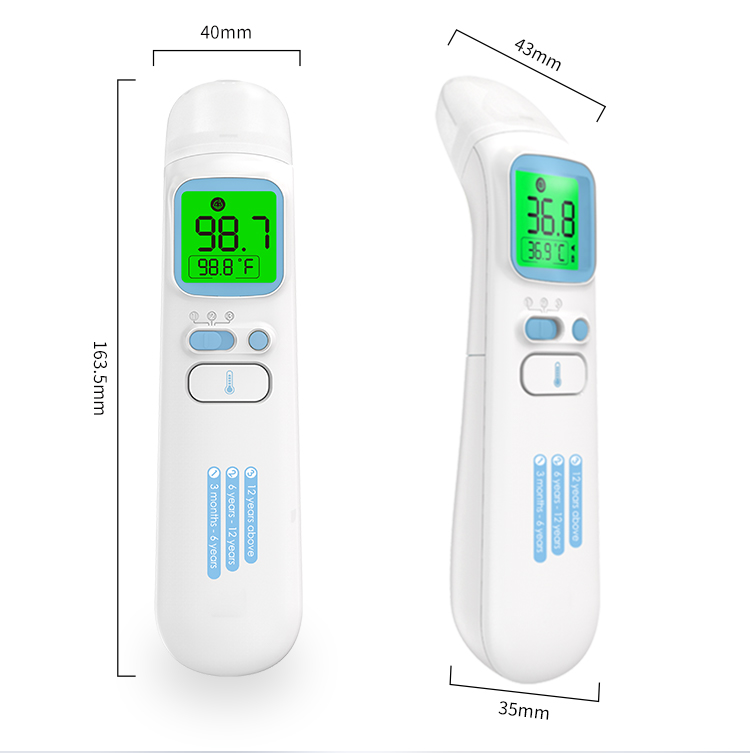
Weight & Dimension |
80g (with battery), 143×35×41mm(body size) |
|